How Many Bonds Can Nitrogen Form
This is because it has atomic number 7 so its electron configuration is 1s22s22p3 giving it 5 valence shell electrons. As known nitrogen could form 3 bonds based on octet rule because it has 5 valence electrons.
Multiple Bonds In Covalent Bonding Dummies
Carbon with 4 single electrons is the most versatile and can form single double and triple covalent bonds maximum of 4 bonds.
. Nitrogen has one lone pair of electrons in its 2s orbital. Nitrogen has 5 electrons 1 pair and 3 singles and can form three single covalent bonds or one triple covalent bond maximum of 3 bonds. How many bonds can nitrogen form.
Nitrogen can form 3 covalent bonds and 1 coordinate bond. Nitrogen has 5 valence electrons and is in a row with a maximum valence number of 8. It cannot accept any more electrons but heres how it forms the fourth bond.
Therefore nitrogen can form 3 single covalent bonds. Nitrogen is one of the few elements that readily forms strong multiple bonds. Either with single double or triple bonds.
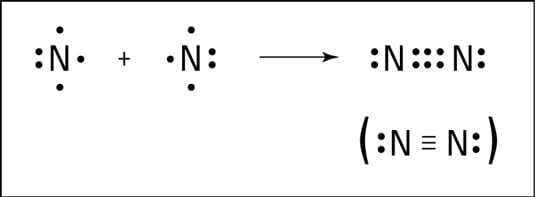
Nitrogen atoms will form three covalent bonds also called triple covalent between two atoms of nitrogen because each nitrogen atom needs three electrons to fill its outermost shellThe carbon atom has four electrons in its outermost shell and needs four more to fill it. Nitrogen atoms will form three covalent bonds also called triple covalent between two atoms of nitrogen because each nitrogen atom needs three electrons to fill its outermost shell. As known nitrogen could form 3 bonds based on octet rule because it has 5 valence electrons.
Therefore it can form three bonds by sharing its three electrons. Dont get confused as in this question single nitrogen atom N is asked not for dinitrogen N_2. 3 single covalent bonds As for the formation of a single covalent bond one electron is shared by each atom thus nitrogen can share its three electrons to form 3 single covalent bonds.
Nitrogen atoms will form three covalent bonds also called triple covalent between two atoms of nitrogen because each nitrogen atom needs three electrons to fill its outermost shell. If you look at the above image you can see that when nitrogen has a positive charge one less electron it can form four covalent bonds. Even though nitrogen has five valence electrons it is unable to form five covalent bonds.
Why nitrogen is N2 but phosphorus as p4. N Atom- 1s2 2s2 2p3 nitrogen cant make excitation like carbon since it has no further vacant orbitals it can form 3 covalent bonds by 2p3 electrons and 1 coordinate covalent bond by 2s2 electrons hence N can form maximum four covalent bonds. Since nitrogen has five valence electrons and bonds it uses three of its five valance electrons for bonding.
Nitrogen has 5 valence electrons and is in a row with a maximum valence number of 8. It is similar to phosphorus in this regard because they both have five valence electrons four when they have a positive charge. Nitrogen is one of the few elements that readily forms strong multiple bonds.
The number of valence electrons an atom possesses determines how many covalent bonds it can form. Nitrogen typically forms 3 covalent bonds including in N 2. That means it needs 3 bonds.
To form a full outer shell of 8 it needs to share 3 electrons forming 3 covalent bonds. It typically forms 3 bonds and has a lone pair NH 3 or makes 4 bonds with a positive charge NH 4. It can actually form 3.
In dinitrogen two nitrogen atoms are bonded with each other by a triple bond as each nitrogen atom shares its. How many bonds can nitrogen form. It can donate this electron pair to form a coordinate bond.
Nitrogen cannot really form 5 bonds unless you count 4 covalent bonds and 1 ionic bond. Normally a nitrogen atom forms 3 bonds but when the nitrogen atom has a positive charge it is deficient in an electron so it can form an additional fourth covalent bond. The top illustration shows a coordinate-covalent bond between a metal ion eg Fe shown in red and a.
How many single covalent bonds can the nitrogen atomic 7 atom form. A nitrogen atom forms three covalent bonds. Nitrogen is in group 5 and therefoe has 5 outer electrons.
It typically forms 3 bonds and has a lone pair NH 3 or makes 4 bonds with a positive charge NH 4. Nitrogen owing to its small size has a tendency to form pπ-π multiple bonds with itselfNitrogen thus forms a very stable diatomic molecule N 2On moving down a group the tendency to form pπ-pπ bonds decreases because of the large size of heavier elements. Nitrogen atoms will form three covalent bonds also called triple covalent between two atoms of nitrogen because each nitrogen atom needs three electrons to fill its outermost shell.
Nitrogen has three electrons in its 2p orbital. That means it needs 3 bonds.

Coordination Compounds How Many Bonds Can Nitrogen Form Chemistry Stack Exchange
Why Does Nitrogen Form 4 Bonds And Oxgen 3 Bonds Can Someone Explain In Terms Of Orbitals Four Bond Formation In Nitrogen And 3 Bond In Oxygen And How Charge Comes

What Are Hybrid Orbitals Master Organic Chemistry Organic Chemistry Organic Chemistry Study Organic Chemistry Books
No comments for "How Many Bonds Can Nitrogen Form"
Post a Comment